Key Points:
- Study compares ability of clinical-diffusion vs MTT-diffusion mismatch to predict infarct growth after stroke
- Clinical-diffusion mismatch is best predictor of infarct growth
Clinical-diffusion mismatch is a good predictor of infarct growth among patients with middle cerebral artery (MCA)-M1 occlusion and small infarct core, according to research published online March 8, 2016, ahead of print in the Journal of NeuroInterventional Surgery. However, MTT-diffusion mismatch is not predictive of infarct growth.
To investigate the predictive abilities of clinical-diffusion and MTT-diffusion mismatch, Raul G Nogueira, MD, of Grady Memorial Hospital (Atlanta, GA), and colleagues conducted a retrospective analysis of 43 stroke patients with MCA-M1 occlusions.
All patients had undergone an MRI within 10 hours of onset and had a diffusion weighted imaging (DWI) volume ≤ 25 mL. Mean age was 75.8 years, and median National Institutes of Health Stroke Scale (NIHSS) score was 13.
The investigators defined clinical-diffusion mismatch as a baseline NIHSS score ≥ 8 and DWI volume ≤ 25 mL. MTT-diffusion mismatch was defined as an MTT lesion volume ([MTT-DWI])/DWI) ≥ 20% and ≥ 10 mL larger than the DWI lesion volume. Significant infarct growth was defined as a > 20% (> 5 mL) increase in infarct volume on follow-up imaging.
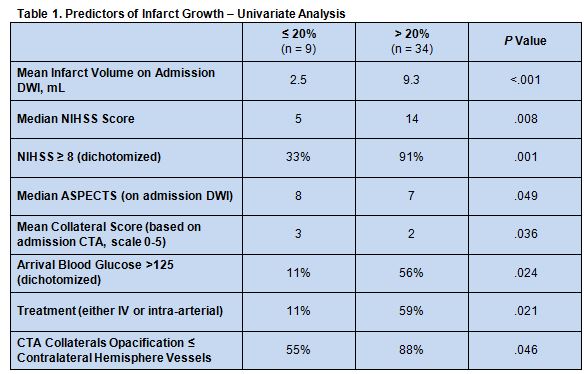
On univariate analysis, multiple admission imaging and clinical variables differed between patients who had infarct growth greater than 20% and those with growth less than or equal to 20% (table 1), but there was no significant association between the amount of MTT-DWI mismatch and infarct growth (P = 0.33). In fact, all patients had MTT-DWI mismatch > 20%.
On multivariate analysis, only baseline NIHSS score ≥ 8—which in this cohort represented the presence of clinical-diffusion mismatch because all patients had a DWI volume ≤ 25 mL—was an independent predictor of stroke growth (OR 20.67, 95% CI 3.34-128.0).
Imaging May Improve Patient Selection and Ultimately Outcomes
The ultimate goal of predicting in infarct growth is to improve patient selection for stroke intervention, Greg Albers, MD, of Stanford University (Stanford, CA), told WLNCMD in a telephone interview. Imaging technology is increasingly being used successfully to estimate the amount of salvageable brain tissue remaining after a stroke, allowing for better identification of patients likely to benefit from reperfusion.
He pointed out that trials that did not make an effort to preselect appropriate patients based on imaging, such as MRCLEAN, achieved good outcomes in only about one-third of patients. In contrast, trials that preselected patients using CT perfusion imaging, such as EXTEND-IA, report good outcome rates as high as 70%.
While this information can be obtained via MRI, many hospitals do not have the infrastructure required to perform that imaging test rapidly in a stroke patient. In such circumstances, CT perfusion must be relied upon, and Dr. Albers was among the developers of the RAPID software that makes this possible. Indeed, this approach proved effective in a recent study.
Use of imaging technology to predict infarct size, said Dr. Albers, represents an important advance from previous efforts to select patients for reperfusion based on time windows, such as the amount of time since the onset of symptoms. The latter is limited by the fact that some strokes complete more quickly than others and because it is impossible to determine onset of symptoms for many patients, such as those whose strokes began while they were asleep. “This is a much more intelligent approach to stroke,” he said. “As we move into the future, this will be the way we go, but we have to be able to offer [imaging] very quickly.” As a result, stroke centers are increasingly making MRI available on an emergent basis.
Source:
- Nogueira RG, Kemmling A, Souza LM, et al. Clinical diffusion mismatch better discriminates infarct growth than mean transit time-diffusion weighted imaging mismatch in patients with middle cerebral artery-M1 occlusion and limited infarct core. J NeuroInterv Surg. 2016;Epub ahead of print
Disclosures:
- Dr. Albers is one of the developers of the RAPID software and principal investigator for the DIFFUSE 3 trial
- Dr. Nogueira is principal investigator for the TREVO-2 and DAWN trials (Stryker Neurovascular), steering committee member of the SWIFT and SWIFT-Prime trials, Core Lab of the STAR Trial (Covidien/ev3 Neurovascular), and executive committee member of the 3D Separator Trial (Penumbra)